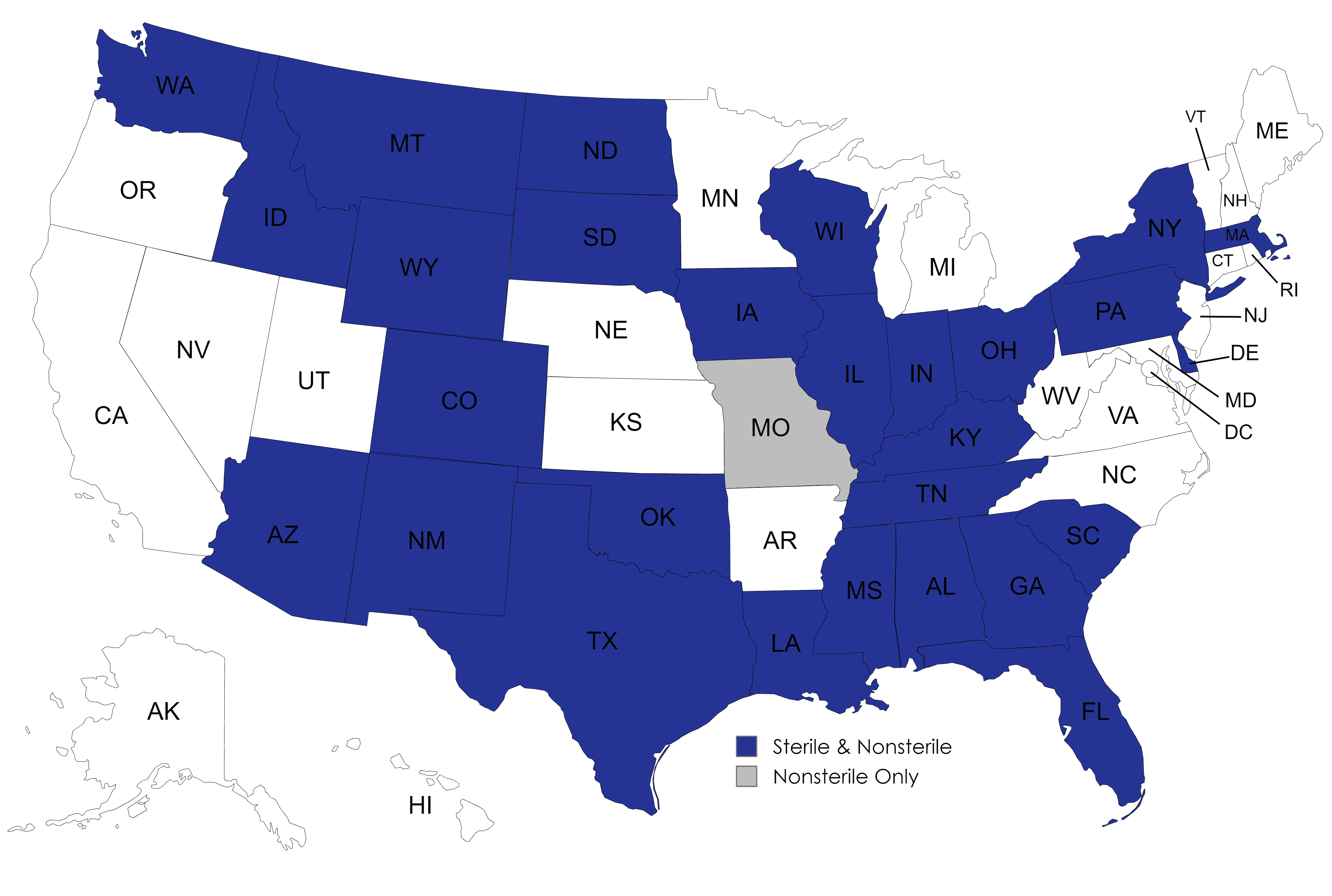
BRD currently ships to veterinarians in the following states.
Veterinarians in these states should click HERE to begin the registration process.
BRD only compounds medications for veterinarians.
Owners and caregivers in these states should contact their veterinarian about our preparations.
*Shipping charges will be applied for any drop shipment orders directly to owners/caregivers
